The gut microbiome is emerging as a key player in women’s health, and could influence everything from the menstrual cycle to fertility, pregnancy and menopause. For healthcare professionals, understanding the bidirectional relationship between our gut microbiome and hormones (known as the ‘gut-hormone axis’) could open up opportunities for microbial-targeted, life-stage specific interventions to support gut, immune and hormone health, thus improving patient outcomes.


By Dr Holly Neill, Science Manager at Yakult UK and Ireland and Lucy McLean ANutr, Science Communications Specialist at Yakult UK and Ireland.
We recently conducted a survey of 2,044 women in the UK, which found that 86% had never heard of the gut-hormone axis, and 4 in 5 women wanted to know more about how gut health affects overall wellbeing.1 This highlights both the knowledge gap and the opportunity for healthcare professionals to provide evidence-based guidance.
What is a “healthy” gut microbiota?
The gut microbiota is the collection of microbes (e.g. bacteria, archaea, fungi and viruses) living throughout the gastrointestinal tract. There is no universal definition of a “healthy” microbiota, in fact our gut microbiota is as unique as our fingerprint. Nevertheless, there are three characteristics that are considered key: diversity, stability and resilience.
What is the gut-hormone axis?
- Sex Hormones: The two main female sex hormones are oestrogen (in forms like estradiol, estrone, estriol) and progesterone. Differences exist between the male and female gut microbiota.2,3 These sex-related differences in the gut microbiota, and how they interact with sex hormones and the immune system, is referred to as ‘microgenderome’.4 Considerably greater gut microbiota diversity is seen in females (at bacterial phyla, genus and species level).5,6,7 However, these differences between sexes lessen throughout life, especially during perimenopause and menopause due to changes in sex hormones. This may, in part, explain why the risk of certain diseases is higher in females compared to men (e.g. autoimmune disease, osteoporosis and irritable bowel syndrome).
- Oestrobolome: This refers to a group of gut bacteria that produce the β-glucuronidase enzyme, which can “reactivate” or “recycle” oestrogen, reducing its elimination and enabling its reabsorption.
How does the gut microbiome influence female health?
Let’s consider some key life stages and how the gut-hormone axis is involved.
Early Life: Birth To Childhood
- Mode of delivery (vaginal or caesarean) and feeding type (breast milk or formula) strongly influence early colonisation of the gut microbiota. Vaginal birth and breastfeeding are linked to higher levels of Bifidobacterium and Bacteroides.8,9
- By the age of 3 years old, the gut microbiota generally stabilises and begins to resemble that of an adult.10
- Key modifying factors during this formative stage include diet, antibiotic use and environment (e.g. living in urban or rural area, hygiene, pollution, siblings and owning a pet). These can shape microbial diversity and long-term health outcomes.11
Puberty to Reproductive Years
- As females enter puberty, fluctuating levels of oestrogen and progesterone begin to influence gut function.
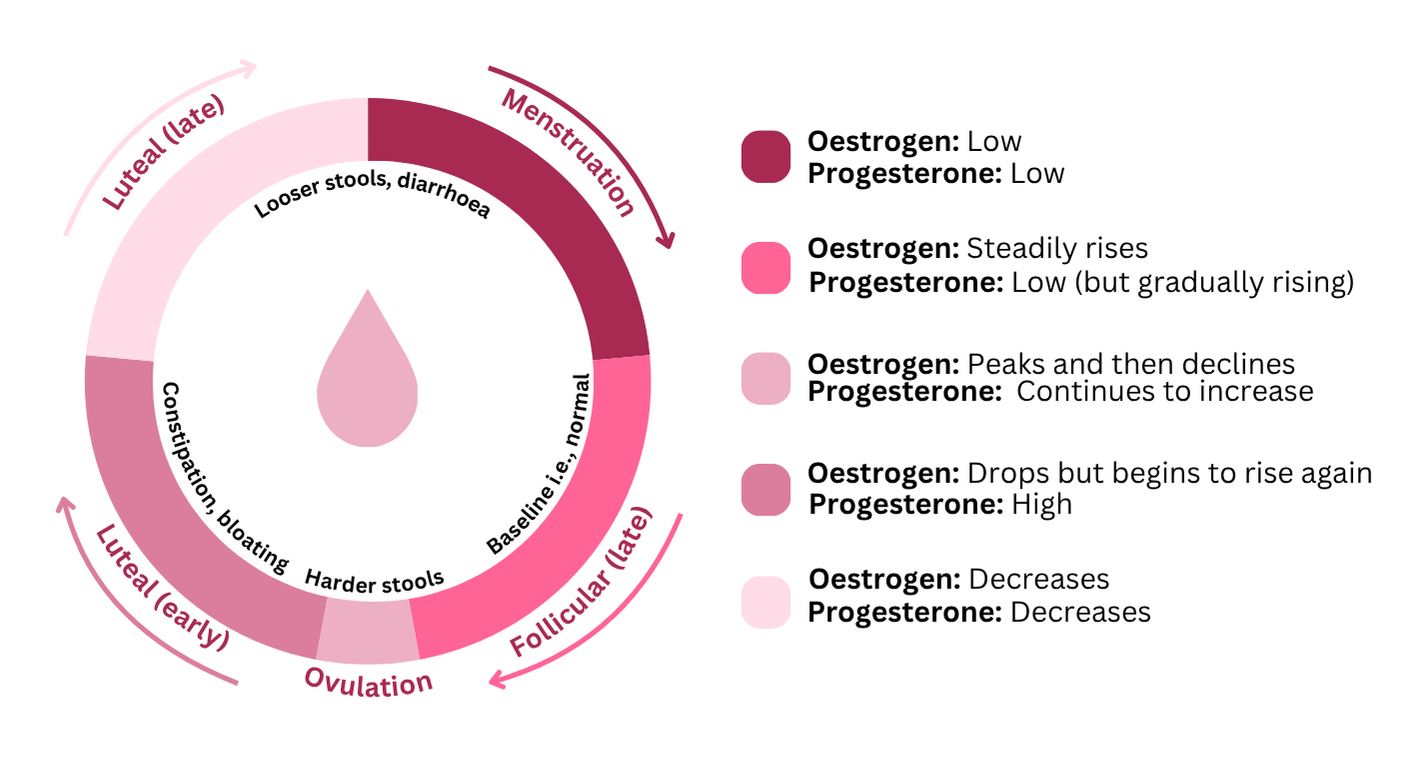
- Throughout the menstrual cycle, many females can experience gastrointestinal symptoms such as bloating, constipation or diarrhoea. These changes are mediated by sex hormone receptors located in gastrointestinal cells, which affect gut motility (i.e. movement of food through the digestive tract) and gut permeability.12 For example, around ovulation, when progesterone levels rise, gut motility may slow, leading to harder stools or constipation. Whereas during menstruation, when progesterone levels drop sharply, gut motility tends to speed up, often resulting in looser stools or diarrhoea. So, yes – ‘period poop’ is a real thing!
- Oral contraceptives: Some small human studies have reported modest differences in gut microbiota composition between users verses non-users.13,14
- Polycystic ovary syndrome (PCOS): A common condition affecting approximately 6-13% of reproductive-aged women.15 It affects how a women’s ovaries work and is characterised by excess levels of androgen, irregular or absent periods, and polycystic ovaries. Lower gut microbiota diversity and dysbiosis (i.e. imbalance) is a common feature of PCOS and may impact disease onset and progression.16,17,18,19 Probiotics (especially those with a Lactobacillus strain) show promising results for improving insulin resistance, reducing inflammation and possibly improving fertility in some women. However, much more research is needed in this area.
- Endometriosis: A chronic inflammatory disease in which tissue similar to the lining of the uterus grows outside of the uterus. It affects roughly 10% of reproductive aged women20 and can cause severe pelvic pain particularly during menstruation, heavy menstrual bleeding, painful periods, pain with bowel movements or urination and can contribute to fertility issues. Some studies have shown alterations in the gut microbiota of those struggling with endometriosis.19,21 Dietary and lifestyle factors, such as a Mediterranean-style diet rich in fibre and polyphenols are known to increase gut microbiota diversity and may help manage symptoms.
Fertility
- Infertility is defined as the inability to become pregnant after 12 or more months of regular unprotected sex and affects around 1 in 7 heterosexual couples.22 Secondary infertility refers to being unable to conceive following a successful pregnancy.
- Several male and female factors, both modifiable and non-modifiable, can affect fertility.
- Gut dysbiosis can promote inflammation and immune dysregulation which can also influence reproductive health. Observational research has found that the gut microbiota is different between fertile and infertile women, however a causation has not been established.23,24,25,26,27,28
- Gut microbiota and vaginal microbiota may impact ovulation, implantation success and pregnancy outcomes such as risk of miscarriage.
Pregnancy
- Gastrointestinal symptoms (e.g. constipation, haemorrhoids) can arise during pregnancy as sex hormone levels change.29,30
- As pregnancy progresses, especially in the later stages when oestrogen levels peak, maternal gut microbial composition shifts and changes in diversity.
- Gut dysbiosis is implicated in pregnancy complications such as gestational diabetes, pre-eclampsia and restricted foetal growth.31,32
Postpartum Period
- Postpartum depression (PPD) affects around 10-15% of women.33
- Emerging observational evidence suggests women with PPD have a different distinct gut microbiota profile compared to those without PPD. The gut-brain axis may be involved via short-chain fatty acids (SCFAs) and tryptophan (the precursor to serotonin, known as the ‘happy hormone’) pathways.34,35
- Some probiotic interventions (e.g. Lactobacillus strains) during pregnancy and postpartum have shown promise in lowering depression and anxiety,36 however strain specificity, timing and dosage remain important considerations which require further research.
Perimenopause, Menopause & Later Life
- The transition to menopause is referred to as ‘perimenopause’ and can last between 1-10 years. It’s characterised by irregular periods, variable bleeding and hormonal fluctuations.
- Menopause (typical onset 45-55 years) describes the cessation of menstruation for 12 consecutive months, accompanied by a decrease in oestrogen and progesterone. Symptoms include hot flushes, mood changes, sleep disturbance, joint discomfort, night sweats and sexual dysfunction.
- Post-menopausal women show reduced gut microbial diversity, changes in bacteria composition (e.g. lower Firmicutes and Ruminococcus, higher Bacteroides, Prevotella, Dorea, Sutterella), decrease in SCFA-producing bacteria and lowered β-glucuronidase potential (i.e. oestrobolome function).37,38 At this stage the female gut microbiome also becomes more similar to males.39,40 Gut microbiome changes may also contribute to metabolic syndrome, bone loss (osteoporosis), cardiovascular risk and mood fluctuations in post-menopausal women.
- Hormone replacement therapy: Emerging research suggests hormone replacement therapy (HRT) may mitigate some gut microbiota alterations (e.g. dysbiosis) associated with menopause, help to restore diversity and potentially improve gut barrier function.41 However, clinical data is limited and more research is required.
How to support your patients gut health
Diet, exercise, sleep, stress management and hydration can have a significant impact on the gut microbiome across life stages and conditions. However, personalisation and recognising personal lived experience are important when providing recommendations to patients.
Key Takeaways for Healthcare Professionals
- The gut microbiome is a complex ecosystem that interplays with female physiology at each life stage.
- Gut dysbiosis is associated with fertility issues, reproductive conditions, pregnancy complications and menopausal symptoms.
- Early life and hormonal transition periods (e.g. puberty, pregnancy, menopause) represent windows of vulnerability and opportunity for microbial-target interventions.
- Diet (e.g. Mediterranean-style diet rich in fibre and polyphenols) and lifestyle interventions (e.g. exercise, sleep, stress management) can support the gut microbiome and subsequently improve patient outcomes across the life cycle.
- Given individual variability, a patient-centred approach should be taken to encourage females to make informed choices and maintain realistic, sustainable changes to improve overall wellbeing.
For too long, women have been underrepresented in research, with biological differences seen as barriers rather than opportunities for greater understanding. Today, more studies are prioritising female health, and emerging research on the gut-hormone axis is providing valuable insights that can empower healthcare professionals to better support and optimise female health across the lifecycle.
Author Bios:
Dr Holly Neill – Holly is the Science Manager at Yakult UK and Ireland where she communicates the latest research on the gut microbiota, probiotics, and health. Holly holds a PhD in nutrition and has experience managing and conducting human trials across a range of topics in both healthy populations and ileostomy patients. Before transitioning to industry, she worked as a postdoctoral researcher and has published her research in high impact journals alongside presenting at both national and international conferences.
Lucy McLean ANutr – Lucy graduated from the University of Leeds in September 2024 with an MSc in Nutrition. She has experience creating evidence-based content and working alongside award-winning nutritionists. Lucy is passionate about translating the science of gut health into accessible content that supports healthier, happier lives.
Disclaimer: This blog was supported by an unrestricted educational grant from the healthcare professional support team at Yakult UK and Ireland. Approval of each sponsor and activity is carefully assessed for suitability on a case by case basis. Sponsorship does not imply any endorsement of the brand by MyNutriWeb, its organisers, its moderators or any participating healthcare professional, or their association. Sponsorship funds are reinvested into the creation and promotion of professional development opportunities on MyNutriWeb
References
- Yakult. Women’s gut health guide. June 17, 2024. Accessed October 13, 2025. http://www.yakult.co.uk/gut-science/womens-gut-guide/
- Jašarević E, Morrison KE, Bale TL. Sex differences in the gut microbiome-brain axis across the lifespan. Philos Trans R Soc Lond B Biol Sci. 2016;371(1688):20150122. doi:10.1098/rstb.2015.0122
- Kim YS, Unno T, Kim BY, Park MS. Sex Differences in Gut Microbiota. World J Mens Health. 2020;38(1):48-60. doi:10.5534/wjmh.190009.
- Vemuri R, Sylvia KE, Klein SL, et al. The microgenderome revealed: sex differences in bidirectional interactions between the microbiota, hormones, immunity and disease susceptibility. Semin Immunopathol. 2019;41(2):265-275. doi:10.1007/s00281-018-0716-7
- Haro C, Rangel-Zúñiga OA, Alcalá-Díaz JF, et al. Intestinal Microbiota is influenced by gender and body mass index. PLoS One. 2016;11(5):e0154090. doi:10.1371/journal.pone.0154090.
- de la Cuesta-Zuluaga J, Kelley ST, Chen Y, et al. Age- and Sex-Dependent Patterns of Gut Microbial Diversity in Human Adults. mSystems. 2019;4(4):e00261-19. Published 2019 May 14. doi:10.1128/mSystems.00261-19
- Mueller S, Saunier K, Hanisch C, et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol. 2006;72(2):1027-1033. doi:10.1128/AEM.72.2.1027-1033.2006
- Korpela K. Impact of Delivery Mode on Infant Gut Microbiota. Ann Nutr Metab. Published online August 30, 2021. doi:10.1159/000518498
- Ma J, Li Z, Zhang W, et al. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: a study of 91 term infants. Sci Rep. 2020;10(1):15792. Published 2020 Sep 25. doi:10.1038/s41598-020-72635-x
- Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222-227. Published 2012 May 9. doi:10.1038/nature11053
- Kumbhare SV, Patangia DVV, Patil RH, Shouche YS, Patil NP. Factors influencing the gut microbiome in children: from infancy to childhood. J Biosci. 2019;44(2):49.
- Bharadwaj S, Barber MD, Graff LA, Shen B. Symptomatology of irritable bowel syndrome and inflammatory bowel disease during the menstrual cycle. Gastroenterol Rep (Oxf). 2015;3(3):185-193. doi:10.1093/gastro/gov010.
- Mihajlovic J, Leutner M, Hausmann B, et al. Combined hormonal contraceptives are associated with minor changes in composition and diversity in gut microbiota of healthy women. Environ Microbiol. 2021;23(6):3037-3047. doi:10.1111/1462-2920.15517.
- Hua X, Cao Y, Morgan DM, et al. Longitudinal analysis of the impact of oral contraceptive use on the gut microbiome. J Med Microbiol. 2022;71(4):10.1099/jmm.0.001512. doi:10.1099/jmm.0.001512.
- World Health Organization (WHO). Polycystic ovary syndrome. February 7, 2017. Accessed October 13, 2025. https://www.who.int/news-room/fact-sheets/detail/polycystic-ovary-syndrome
- Torres PJ, Siakowska M, Banaszewska B, et al. Gut Microbial Diversity in Women With Polycystic Ovary Syndrome Correlates With Hyperandrogenism. J Clin Endocrinol Metab. 2018;103(4):1502-1511. doi:10.1210/jc.2017-02153.
- Guo J, Shao J, Yang Y, et al. Gut Microbiota in Patients with Polycystic Ovary Syndrome: a Systematic Review. Reprod Sci. 2022;29(1):69-83. doi:10.1007/s43032-020-00430-0.
- Lindheim L, Bashir M, Münzker J, et al. Alterations in Gut Microbiome Composition and Barrier Function Are Associated with Reproductive and Metabolic Defects in Women with Polycystic Ovary Syndrome (PCOS): A Pilot Study. PLoS One. 2017;12(1):e0168390. Published 2017 Jan 3. doi:10.1371/journal.pone.0168390.
- Li C, Cheng D, Ren H, Zhang T. Unraveling the gut microbiota’s role in PCOS: a new frontier in metabolic health. Front Endocrinol (Lausanne). 2025;16:1529703. doi:10.3389/fendo.2025.1529703.
- World Health Organization (WHO). Endometriosis. March 24, 2023. Accessed October 13, 2025. https://www.who.int/news-room/fact-sheets/detail/endometriosis
- Yuan M, Li D, Zhang Z, Sun H, An M, Wang G. Endometriosis induces gut microbiota alterations in mice. Hum Reprod. 2018;33(4):607-616. doi:10.1093/humrep/dex372.
- NHS. Infertility. August 11, 2023. Accessed October 13, 2025. https://www.nhs.uk/conditions/infertility/.
- Li T, Shao W, Wang Y, et al. A two-sample mendelian randomization analysis investigates associations between gut microbiota and infertility. Sci Rep. 2023;13(1):11426. doi:10.1038/s41598-023-38624-6.
- Lundy SD, Sangwan N, Parekh NV, et al. Functional and Taxonomic Dysbiosis of the Gut, Urine, and Semen Microbiomes in Male Infertility. Eur Urol. 2021;79(6):826-836. doi:10.1016/j.eururo.2021.01.014.
- Ding N, Zhang X, Zhang XD, et al. Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes. Gut. 2020;69(9):1608-1619. doi:10.1136/gutjnl-2019-319127.
- Kaltsas A, Zachariou A, Markou E, Dimitriadis F, Sofikitis N, Pournaras S. Microbial Dysbiosis and Male Infertility: Understanding the Impact and Exploring Therapeutic Interventions. J Pers Med. 2023;13(10):1491. Published 2023 Oct 13. doi:10.3390/jpm13101491.
- Komiya S, Naito Y, Okada H, et al. Characterizing the gut microbiota in females with infertility and preliminary results of a water-soluble dietary fiber intervention study. J Clin Biochem Nutr. 2020;67(1):105-111. doi:10.3164/jcbn.20-53.
- Wang Y, Xie Z. Exploring the role of gut microbiome in male reproduction. Andrology. 2022;10(3):441-450. doi:10.1111/andr.13143.
- Gomes CF, Sousa M, Lourenço I, Martins D, Torres J. Gastrointestinal diseases during pregnancy: what does the gastroenterologist need to know?. Ann Gastroenterol. 2018;31(4):385-394. doi:10.20524/aog.2018.0264.
- Oh JE, Kim YW, Park SY, Kim JY. Estrogen rather than progesterone cause constipation in both female and male mice. Korean J Physiol Pharmacol. 2013;17(5):423-426. doi:10.4196/kjpp.2013.17.5.423
- Turjeman S, Collado MC, Koren O. The gut microbiome in pregnancy and pregnancy complications. Curr Opin Endocr Metab Res. 2021;18:133-138. doi:10.1016/j.coemr.2021.03.004.
- Zakaria ZZ, Al-Rumaihi S, Al-Absi RS, et al. Physiological Changes and Interactions Between Microbiome and the Host During Pregnancy. Front Cell Infect Microbiol. 2022;12:824925. Published 2022 Feb 21. doi:10.3389/fcimb.2022.824925.
- NHS. Overview – postnatal depression. July 8, 2025. Accessed October 13, 2025. https://www.nhs.uk/mental-health/conditions/post-natal-depression/overview/
- Li S, Zhang X, Cai Y, Zheng L, Pang H, Lou L. Sex difference in incidence of major depressive disorder: an analysis from the Global Burden of Disease Study 2019. Ann Gen Psychiatry. 2023;22(1):53. Published 2023 Dec 12. doi:10.1186/s12991-023-00486-7.
- Liu Z, Li L, Ma S, et al. High-dietary fiber intake alleviates antenatal obesity-induced postpartum depression: Roles of gut Microbiota and microbial metabolite short-chain fatty acid involved. J Agric Food Chem. 2020;68(47):13697-13710. doi:10.1021/acs.jafc.0c04290.
- Slykerman RF, Hood F, Wickens K, et al. Effect of Lactobacillus rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: A Randomised Double-blind Placebo-controlled Trial. EBioMedicine. 2017;24:159-165. doi:10.1016/j.ebiom.2017.09.013.
- Peters BA, Lin J, Qi Q, et al. Menopause Is Associated with an Altered Gut Microbiome and Estrobolome, with Implications for Adverse Cardiometabolic Risk in the Hispanic Community Health Study/Study of Latinos. mSystems. 2022;7(3): e0027322. doi:10.1128/msystems.00273-22.
- Santos-Marcos JA, Rangel-Zuñiga OA, Jimenez-Lucena R, et al. Influence of gender and menopausal status on gut microbiota. Maturitas. 2018;116:43-53. doi:10.1016/j.maturitas.2018.07.008.
- Peters BA, Santoro N, Kaplan RC, Qi Q. Spotlight on the Gut Microbiome in Menopause: Current Insights. Int J Womens Health. 2022;14:1059-1072. Published 2022 Aug 10. doi:10.2147/IJWH.S340491.
- Mayneris-Perxachs J, Arnoriaga-Rodríguez M, Luque-Córdoba D, et al. Gut microbiota steroid sexual dimorphism and its impact on gonadal steroids: influences of obesity and menopausal status. Microbiome. 2020;8(1):136. Published 2020 Sep 20. doi:10.1186/s40168-020-00913-x.
- Jiang L, Fei H, Tong J, et al. Hormone Replacement Therapy Reverses Gut Microbiome and Serum Metabolome Alterations in Premature Ovarian Insufficiency. Front Endocrinol (Lausanne). 2021;12:794496. Published 2021 Dec 23. doi:10.3389/fendo.2021.794496.







